Vx
Home > Products > SphygmoCor > Vx
SphygmoCor® Vx
The SphygmoCor Vx Pulse Wave Velocity System non-invasively measures the speed (m/s) of the pressure wave between two locations in the arterial tree. The pressure wave travels faster through a stiffer vessel, therefore Pulse Wave Velocity is a very important measure of the stiffness of that arterial segment.
 Arterial stiffness is now recognised as a major driver of cardiovascular
disease. An increase in arterial stiffness elevates central systolic and
pulse pressure – as well as left ventricular afterload – and decreases
coronary artery perfusion pressure. These effects increase the risk of
stroke, heart failure and myocardial infarction.
Pulse Wave Velocity is a well established technique for measuring the
arterial stiffness of an arterial segment. Most commonly it is performed
between the carotid and femoral artery sites, to primarily measure the
stiffness of the aorta.
The SphygmoCor Vx Pulse Wave Velocity System is offered as an
add-on option for the SphygmoCor Px Pulse Wave Analysis System.
It uses a 3-lead ECG in conjunction with a tonometer to measure the
pressure pulse waveform sequentially in two peripheral artery sites.
Arterial stiffness is now recognised as a major driver of cardiovascular
disease. An increase in arterial stiffness elevates central systolic and
pulse pressure – as well as left ventricular afterload – and decreases
coronary artery perfusion pressure. These effects increase the risk of
stroke, heart failure and myocardial infarction.
Pulse Wave Velocity is a well established technique for measuring the
arterial stiffness of an arterial segment. Most commonly it is performed
between the carotid and femoral artery sites, to primarily measure the
stiffness of the aorta.
The SphygmoCor Vx Pulse Wave Velocity System is offered as an
add-on option for the SphygmoCor Px Pulse Wave Analysis System.
It uses a 3-lead ECG in conjunction with a tonometer to measure the
pressure pulse waveform sequentially in two peripheral artery sites.
Features and Benefits
- The SphygmoCor Px/Vx System provides a comprehensive assessment of arterial stiffness and the clinical impact of arterial stiffening on key central parameters driving cardiovascular risk
- It is a simple-to-use doctor’s office Pulse Wave Velocity System, with automated software analysis and database facilitiesk
- The pulse waveform recordings and ECG recordings are extensively evaluated to ensure consistent quality
- The system can be used on any two accessible arterial sites
- Only one operator is required to use the system
System Specifications
SphygmoCor® Pulse Wave Velocity System: Model SCOR-Vx
Standard system configuration
- SphygmoCor signal processing electronics module
- SphygmoCor software system
- SphygmoCor pressure tonometer
- System documentation
- ECG Cable & Leads
Performance and operating specifications
-
Analysis data
- User selectable proximal and distal artery sites
- Pulse Wave Velocity with standard deviation
- Heart Rate
- Graph of ΔTs for each set of pulse waves
- Option of using 4 different Algorithms to determine pulse wave trigger points on pressure waveforms:
- Max dP/dT
- Pulse Height %
- Max 2nd Derivative
- Intersecting Tangents
- Quality Control Parameters for raw signal recordings
- Quality Control Algorithms
- Timing data
- Statistical Analysis table provides mean and standard deviation data
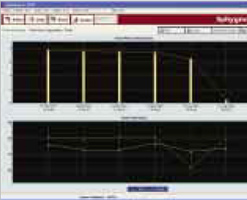
- User selectable graphs showing the trend data for serial patient studies:
- Pulse Wave Velocity
- ΔTs and their standard deviations
- Heart Rate
- Standard patient report includes:
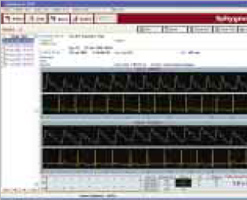
- 10, 20, or 30 seconds of each simultaneous pressure ECG waveform recording
- Graph of ΔTs for each pulse wave
- Quality Control Parameters
- Statistical tables
- Pulse Wave Velocity with standard deviation
- Systolic, diastolic, mean pressures as entered by the operator
- Trigger points marked for each pressure – ECG waveform pairs
The recorded pressure waveforms are calibrated using the brachial pressures as measured by conventional cuff sphygmomanometry. The blood pressures are reported for reference only.
Software features
- Integrated with SphygmoCor Px Pulse Wave Analysis System
- Patient database in Microsoft® Access format
- Improved algorithm for detecting ECG timing points
- Export Function allows data to be readily analyzed with Excel, SPSS, etc
- Import measurements from previous versions of SphygmoCor PWV software
- Up to twenty patient databases can be set up for different clinical studies
- Batch printing of selected reports
- Two capture windows for real-time data capture featuring:
- Auto-scaling of peripheral pressure and ECG waveforms
- Display of the entire user selected capture time of data
- Patient listing facility – to view and print last time a patient undertook a study
- IBM compatible PC: Pentium III/Celeron Processor 400 MHz; 128 MB RAM; 800x600 256 color SVGA Display; 100 MB initial free hard disk space (more for data storage), CD-ROM drive
- Equipment interface: RS-232 serial or USB port
- Operating System: Windows 98SE/ME/2000/XP
- Printing: Windows Compatible Printer
- Ambient temperature: 15-30°C
- Relative humidity: 20-80%
- 220-240 VAC, 50 Hz
- 100-110 VAC, 50/60Hz
- 12VA
- FDA 510K
- EU CE Mark (MDD, ANNEX II, Class IIa)
- MHLW, Japan
- TGA, Australia
- IEC 60601-1/ AS/NZS 3200.1 (amendments 1 and 2) Electromedical Equipment Safety standard
- IEC 60601-1-2 Electro-Medical Equipment, ElectroMagnetic Compliance (EMC) Standard

